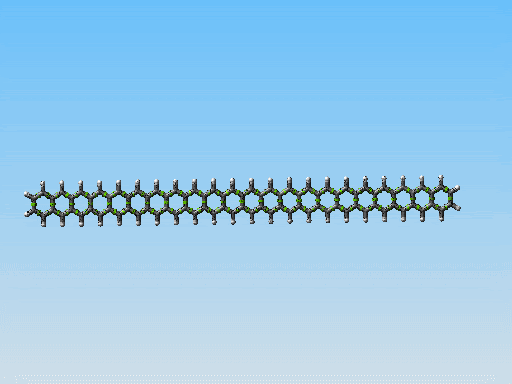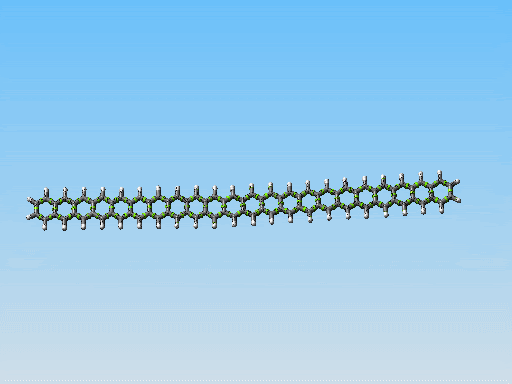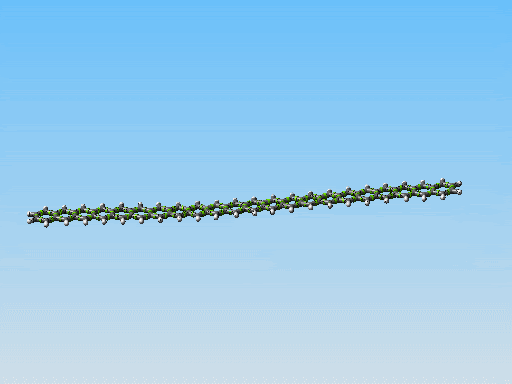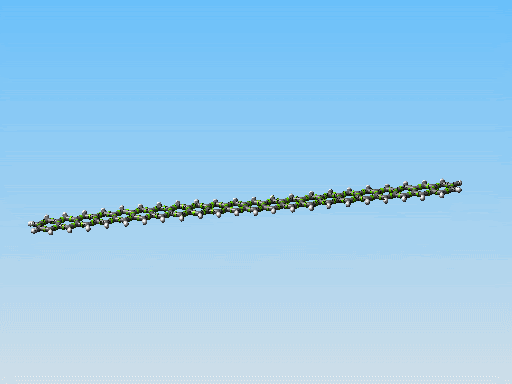
This polycyclic aromatic chain is the stiffest/thinest thing I have come up with yet. Perhaps it could be made to work. However I am starting to consider something else. Take a look at this picture:

I am starting to get a nice little library of diamond-type stuff that is pretty much scaled to work with each other. So I am thinking of rebuilding the Fine Motion Controller at a scale where I can use these cams, gears, rods, etc. In fact I am thinking of making all sorts of similar scaled parts, out of diamond wherever I can, then I will have all these ready made parts that can work with each other. Oh yeah, of course I would offer to add them to the NE1 part library too, then you could have a set as well.

4 comments:
Linear fused ring systems like the one you display there are very unstable past pentacene. Of course, a molecular mechanics simulation would not show that -- yet another reason that it is hard to rely on molecular mechanics simulations alone.
Polyphenanthrenes are far more stable. There's a bunch of literature on this. There are even reasonably good conventional synthetic pathways, though very long chains are hard to build because they become insoluble. That should not be an issue for mechanosynthetically produced chains, of course.
Oh, and it is probably quite sensible to make fully unstrained pure diamondoid parts -- they may be the simplest things to build with primitive mechanosynthesis.
I so knew you were going to post that first one.
Hey, being a good engineer means, first and foremost, understanding your tools and materials. Sadly, getting a feel for organic chemistry and such is hard work, and involves lots of stuff that won't be of direct interest to you.
If you want to get a feel for the issues with linear and non-linear fused phenyl ring systems, I'd suggest looking at the section of an orgo text on aromatic systems, and then maybe look at counting how many configurations the bonds in phenanthrene can assume vs. the bonds in anthracene. At that point, the issue will be dead obvious.
You're not alone in lacking intuition for it, though. A lot of professional organic chemists have wasted years on long fused ring systems because they have too much contempt for theory.
Post a Comment